As we approach winter, depending upon which part of the country we live, we will encounter freezing temperatures. Most of the country will enjoy the poetry and beauty - and the needed respites - of the ice and snow of this special season. (Our friends in Southern California are generally denied the poetry of winter unless they brave the stresses of the freeways to reach the mountains. But they can also observe the essentials of this article by watching an ice cube in a glass of water. Our friends in the Southern Hemisphere experienced what we are going to be talking about six months ago.)
Have you ever noticed that ice floats? Why?
Virtually every material substance contracts when it cools. As it gets warmer, the molecules increase their vibrational energy and require more room: the substance therefore expands as it warms. And, conversely, it contracts as it cools. Materials decrease in volume as they get colder.
Water is the astonishing exception. It expands when it freezes into a solid. That's why lakes freeze from the top down: otherwise life as we know it would be impossible. Why does water violate this general rule? Why does water expand when freezing?
The water molecule is a (not-so-simple) combination of two atoms of hydrogen bonded to one of oxygen. Yet this particular combination possesses an amazing array of unique characteristics that distinguish it from any other material known!
The Freezing Process
Although almost all materials decrease in volume as they get colder, water has an astonishing characteristic. As it drops toward its freezing point of 0oC (32 o F), its volume also reduces until it reaches 4oC, after which it actually increases. This "inverse convection" has the salutary effect of bringing oxygen dissolved at the surface down to the lower depths for use by fish and other organisms.
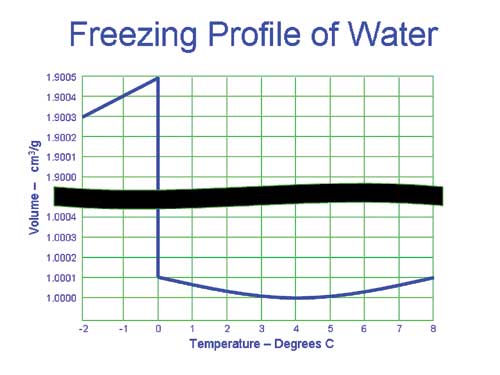
This process continues until the entire area has reached 4oC. As the water cools below this temperature, it dramatically increases in volume, making it lighter than the water below. This ultimately causes the top layer to freeze, which then actually acts as an insulation layer against the very low temperature of the air above.
If water did not have this strange property, the entire pond or lake would freeze solid and fish and other living creatures would be killed, since most animals would have their cells disrupted by the needlelike ice crystals that form in the water within their cells.
The ice has a density 9% less than water and therefore floats. (That's also why icebergs have 90% of their volume below sea level.)
This expansion can have disastrous effects on uninsulated water pipes in winter. However, this expansion effect has essential functions in nature. The rain or dew penetrates the soil, and when it freezes, the soil is shattered into small particles, breaking up the hard earth into suitable conditions in which seeds can germinate.
Why This Exceptional Behavior?
This strange behavior derives from the unusual bonding relationship between the two hydrogen atoms and the one oxygen atom that make up a molecule of water, H2O. The oxygen atom strongly attracts the single electrons of the two hydrogen atoms, leaving the two positively charged hydrogen nuclei rather free to attract other negative atoms. This attracts the oxygen molecules in other water molecules to form rather large, but loosely coupled, frameworks.
These atoms are not in a straight line, however, and the hydrogen atoms are bent toward each other, forming an unsymmetrical three-dimensional structure. The angle formed between the two hydrogen and the central oxygen atom is 104.5o, almost precisely that of a hexagonal tetrahedron shape (109.5o), so it can take up this shape (slightly warped three-dimensionally) with little stress on the bonds. Opposite the hydrogen atoms, the clouds of resulting negative electrification attract the hydrogen nucleus of an adjacent water molecule to form what is called a hydrogen bond - the key to water's peculiar behavior.
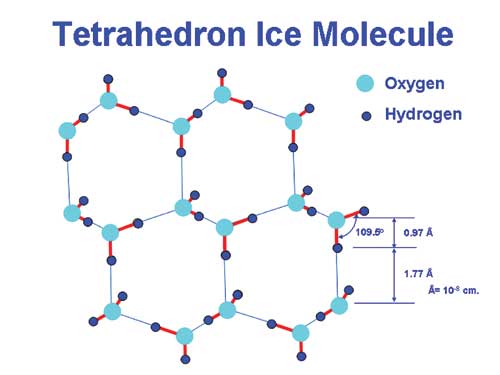
These tenacious hydrogen bond frameworks give water many astonishing characteristics, including anomalously high values for viscosity, surface tension, and the temperature and heats of melting and boiling. This results in its ubiquitous role as a solvent, its remarkable thermal properties (the bane of every Naval Academy Midshipman in his sophomore year), its surface tension and capillary action, and virtually innumerable chemical properties that are essential for life.
Many theories have been developed to explain the structure of pure liquid water: some can account for most of water's peculiar properties, but none can account for all. Certain theories envision the liquid as consisting of a partially broken-down lattice; others suggest a continuum model; others project a mixture model of flickering clusters, etc. The exact nature of the clusters, hydration density, and other aspects are still subjects of controversy, even today.
One would expect that these unique properties would make it a very rare molecule, produced only with great difficulty under laboratory conditions. The reality is, of course, quite the opposite: it is the most prevalent material on the planet, covering of its surface, diffused extensively in the atmosphere, and - to the surprise of the geologists - also found at astonishing depths in the earth. That such a unique substance should be in such abundance is in contradiction to any expectation of random chance alone.
When frozen, the tetrahedron lattice becomes solid, and because of its open shape, it is lighter than water and floats. The molecular structure of ice does not allow other chemicals to be included, which is why sea water can be desalinated by freezing and removing the ice, leaving the salt and other impurities behind.
Snow
Water vapor is a clear gas, which, as it cools under normal conditions, condenses and forms into water droplets. At high altitudes, water vapor can cool to below freezing, but in the absence of an impurity such as dust, around which it can collect, it will remain in this state.
When ice crystals form, the molecules of water arrange themselves in a specific pattern that is determined by the tetrahedral shape of the molecule in the frozen state described above. As further molecules join those already frozen, they give up their high latent heat of freezing, and melt the adjacent molecules, which reform to a shape dependent upon the local conditions of air temperature, wind currents, humidity, etc. Each snowflake pattern is unique to itself, but is always based upon the hexagonal bonding pattern of the ice crystals familiar to us all.
Snow also has a constructive role in the ecological cycle. It filters dust out of the air, absorbs nitrogen which then enters the soil, and acts as an insulating blanket to the plants and roots in the ground. The difference in temperature between the air and the ground covered by two feet of snow can be as much as 40oC.
When snow melts, it requires considerable heat to effect this, and therefore melts slowly, lowering the rate of melt water and reducing the flooding that could occur if the latent heat of freezing were lower.
In addition to all these unique properties, snow also has the added ability of reflecting all the colors of the spectrum to yield pure white. Who can help but be awed by the sheer beauty of a pristine carpet of snow covering a glorious landscape? And soothed by the breathtaking silence of fresh snow lacing the once-familiar trees and fences?
It is astonishing to observe materialistic skeptics purposely ignoring the pervasive evidences of skillful, intelligent design in every aspect of the reality we find ourselves in. This exposes their real motive, which is not the seeking of truth, but the avoidance of accountability to God. I personally am convinced that snow also evidences the sense of humor of our Creator: I believe it was invented for kids to play in - kids of all ages... uh... I have to go now... my grandkids are calling, and we have snowmobiles to untrailer.
Have fun: that's what it's for. And remember that every good thing comes from His hand! Our capacity to enjoy it is, in itself, a gift from Him! Best of the season to you all!
Bibliography
Bowden, Malcolm, True Science Agrees with the Bible, Sovereign Publications, Bromley, Kent, England, 1998.
Encylopaedia Britannica, Vol 12, p.514-515; Vol 15, pp.774-778.






